
Quantitative Analysis of Trimethylamine Oxide and Related Metabolites
Quantitative Analysis of Energy Metabolism
Quantitative Analysis of Short-Chain Fatty Acids
Quantitative Analysis of Fatty Acids
Quantitative Analysis of Bile Acids
Quantitative Analysis of Trimethylamine Oxide and Related Metabolites
Quantitative Analysis of Amino Acids
Quantitative Analysis of Neurotransmitters
Quantitative Analysis of Organic Acids
Quantitative Analysis of Flavonoids
Quantitative Analysis of Carbohydrates
Quantitative Analysis of Plant Hormones
Quantitative Analysis of Carotenoids
Quantitative Analysis of Tannins
Quantitative Analysis of Phenolic Acids
Quantitative Analysis of Anthocyanins
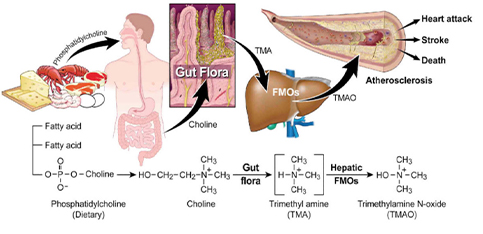
Trimetlylamine oxide (TMAO) is a small molecule compound widely found in aquatic products in nature, also exists in mammals, plants and fungi. It can participate in many important biological functions of the body, such as osmotic pressure regulation , maintain cellular homeostasis, etc. In recent years, with the depth study of intestinal flora, scientists have found that the levels of TMAO and related metabolites are closely related to cardiovascular disease, myocardial infarction, stroke, diabetes, chronic kidney disease, cancer and other diseases. Therefore, TMAO has received more and more attention, and related studies have been reported.
Journal: Nature medicine Impact factor: 36.13 Published date: September, 2018 Published by: Cleveland Lerner Institute, United States
Trimethylamine N-oxide (TMAO) is a gut microbiota–derived metabolite that enhances both platelet responsiveness and in vivo thrombosis potential in animal models, and TMAO plasma levels predict incident atherothrombotic event risks in human clinical studies. TMAO is formed by gut microbe–dependent metabolism of trimethylamine (TMA) moiety-containing nutrients, which are abundant in a Western diet.
TMA-generating enzyme pair, CutC and CutD (CutC/D), we developed inhibitors that are potent, time-dependent, and irreversible and that do not affect commensal viability. In animal models, a single oral dose of a CutC/D inhibitor significantly reduced plasma TMAO levels for up to 3 d and rescued diet-induced enhanced platelet responsiveness and thrombus formation, without observable toxicity or increased bleeding risk. The inhibitor selectively accumulated within intestinal microbes to millimolar levels, a concentration over 1-million-fold higher than needed for a therapeutic effect.
These studies reveal that mechanismbased inhibition of gut microbial TMA and TMAO production reduces thrombosis potential, a critical adverse complication in heart disease. They also offer a generalizable approach for the selective nonlethal targeting of gut microbial enzymes linked to host disease limiting systemic exposure of the inhibitor in the host.

The platelet activity index of mice did not change, but TMAO level and aggregation reaction increased sharply. DMB could reverse TMAO level and ADP-dependent platelet aggregation in mice fed a cholinergic diet, however, TMAO levels and platelet aggregation were not reversed after direct injection of TMAO.
DMB could significantly prolong the time of blood spot formation, but DMB was not effective after TMAO direct injection.
Development of two potent inhibitors (IMC and FMC) that do not affect the viability of symbiotic bacteria but permanently inactivate CutC/D, a key enzyme for TMA production in bacteria.
Accumulates in intestinal commensal bacteria, almost inhibit the production of TMA and TMAO caused by high choline diet completely, reduce platelet aggregation and thrombosis, and no obvious toxic side effects were observed.
IMC and FMC significantly increased the abundance of akermann bacteria in the gut of cholinergic mice
These studies suggest a new potential target for the treatment of subjects at risk for thrombotic complications and cardiovascular disease.
Roberts Adam B,Gu Xiaodong,Buffa Jennifer A et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential.[J] .Nat. Med., 2018, 24: 1407-1417
 © Copyright 2015-2022 Suzhou PANOMIX Biomedical Tech Co.,Ltd
© Copyright 2015-2022 Suzhou PANOMIX Biomedical Tech Co.,Ltd