
Metabolic Flux Analysis
Quantitative Analysis of Energy Metabolism
Quantitative Analysis of Short-Chain Fatty Acids
Quantitative Analysis of Fatty Acids
Quantitative Analysis of Bile Acids
Quantitative Analysis of Trimethylamine Oxide and Related Metabolites
Quantitative Analysis of Amino Acids
Quantitative Analysis of Neurotransmitters
Quantitative Analysis of Organic Acids
Quantitative Analysis of Flavonoids
Quantitative Analysis of Carbohydrates
Quantitative Analysis of Plant Hormones
Quantitative Analysis of Carotenoids
Quantitative Analysis of Tannins
Quantitative Analysis of Phenolic Acids
Quantitative Analysis of Anthocyanins
Metabolomics reflects the static abundance ratio of metabolites, and the increase of a single metabolite may be due to either the active synthetic pathway or the inhibition of the depletion pathway. Therefore, metabonomics is often inadequate to explain all the problems in the study of specific metabolic pathways and metabolic networks.
As a complement to metabonomics, Metabolic Flux analysis uses stable isotope to label specific molecules and track their metabolism in an organism, thus providing dynamic information on the Metabolic pathways of metabolites.
Tumor metabolism mechanism
Immune-related diseases
Metabolic disease
Stem cell development and differentiation
Microorganism
Simulate biological processes Bacterial metabolitesPlants
Resilience Crop quality BreedingAnimal
Animals’ nutrition>Quality of meat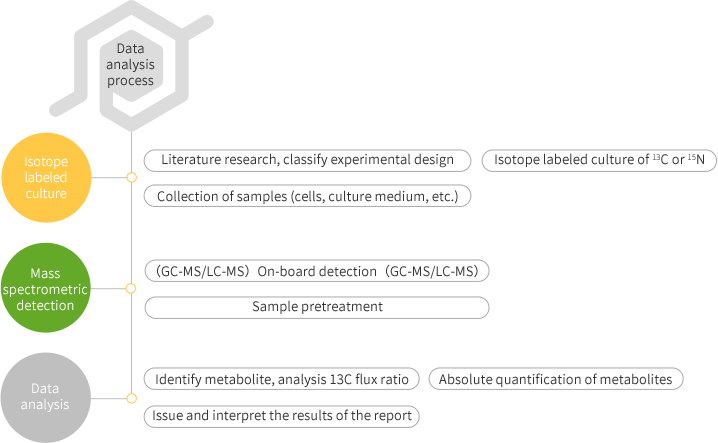
journal: Annals of the rheumatic diseases Impact factor: 16.102 Published date: July 2019 Published by: University of Paris, France
Deciphering the role of glycolysis and oxidative phosphorylation in IL-1β-induced microcrystal responses.
Macrophages were stimulated with monosodium urate (MSU) and calcium pyrophosphate (CPP) crystals and subjected to in vitro metabolomics and real-time extracellular flux analysis to observe the metabolic phenotype of macrophages. Using f-deoxyglucose positron emission tomography, glucose uptake experiments, and molecular biological methods that inhibit glucose invertase (Glut1) , evaluation of the effects of aerobic glycolysis on IL-1 β production in vitro and in vivo.
We observed that MSU and CPP crystals led to metabolic reorganization of macrophages into the aerobic glycolytic pathway, which is caused by increased glucose uptake and expression of GLUT1 plasma membrane in macrophages. Furthermore, neutrophils isolated from human synovial fluid during gout flares express GLUT1 more frequently than neutrophils isolated from blood. Both 2-deoxyglucose or GLUT1 inhibited NLRP3 activation and IL-1β production, thereby inhibiting microcrystalline inflammation.
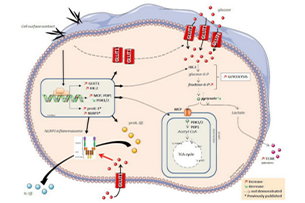

GLUT1-mediated glucose uptake plays an important role in MSU- and CPP crystal-induced IL-1β protein depletion responses. These findings open new avenues for the treatment of arthritis in both acute and chronic patients.
Renaudin Felix,Orliaguet Lucie,Castelli Florence et al. Gout and pseudo-gout-related crystals promote GLUT1-mediated glycolysis that governs NLRP3 and interleukin-1β activation on macrophages.[J] .Ann. Rheum. Dis., 2020
 © Copyright 2015-2022 Suzhou PANOMIX Biomedical Tech Co.,Ltd
© Copyright 2015-2022 Suzhou PANOMIX Biomedical Tech Co.,Ltd