There is a complex neuro-endocrine network between the brain and the gut, which connects the brain and the gastrointestinal tract, so it is called the gut-brain axis. The gut-brain axis is a bidirectional regulatory axis for the interaction of gastrointestinal function with the central nervous system. Among them, the neuro-endocrine-immune network is an important connection mode. Gut-brain neuroendocrine cells are regulated by the central nervous system, enteric nerve and autonomic nervous system, and maintain the normal operation of the gut-brain axis by secreting neurotransmitters or related hormones. Gut microbes play a vital role in the metabolism of nutrients, the body's own development, immunity and the production of diseases, the appearance of gut-brain axis provides new ideas and measures for the prevention and treatment of diseases. After entering the era of data and information, the conventional research methods for intestinal microbes are gradually replaced by the combination of high-throughput and multi-omics.
The multi-omics study of gut microbes can explore the gene expression interaction pattern of the gut microbe hosts through the detection of microbial diversity at the transcriptome level, and then detect the enzyme activity according to the proteome experiment, and investigate the catalytic reaction mechanism of digestion, and the biosynthetic pathway and the final metabolites were further explored by means of metabonomics to analyze the digestion and absorption functions.
Journal: Cell Reports Impact factor: 8.109 Published date: March, 2018 Published by: Harvard Medical School
Diet, genetics and gut microbiota are the main factors affecting metabolism.
This study select mice of different strains with different susceptibility to diabetes and obesity and fed a high-fat diet (HFD) respectively. At the same time, they were given antibiotics such as vancomycin and metronidazole. 16S rRNA genome sequencing was used to investigate the influence of cecal gut microbiota structure and function; and the changes of metabolites in caecum and plasma were investigated by untargeted metabonomics respectively;The relationship between metabolism and insulin resistance in plasma and the relationship between intestinal microflora and insulin resistance were analyzed. It was confirmed that the interaction among diet, gene and intestinal microflora affected the level of metabolites in plasma, to elucidate the pathogenesis of insulin resistance and metabolic syndrome.
The 16S sequence of PICRUST was used to predict the metagenome of intestinal bacteria, revealing significant differences in Microbial metabolism. Cecum and plasma metabolites show multiple differences, reflecting the combined effects of diet, antibiotics, host background, and gut microbiota. There were positive or negative correlations between 18 plasma metabolites and host insulin resistance in different strains and diets. More than 1,000 undetermined metabolic peaks are also highly regulated by diet, antibiotics, diet, and genetic background.
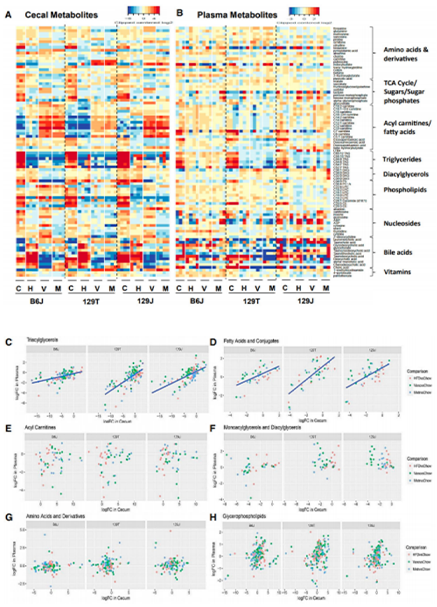 Figure 1 Alterations in gut microbiota affect cecal and plasma metabolites
Figure 1 Alterations in gut microbiota affect cecal and plasma metabolites
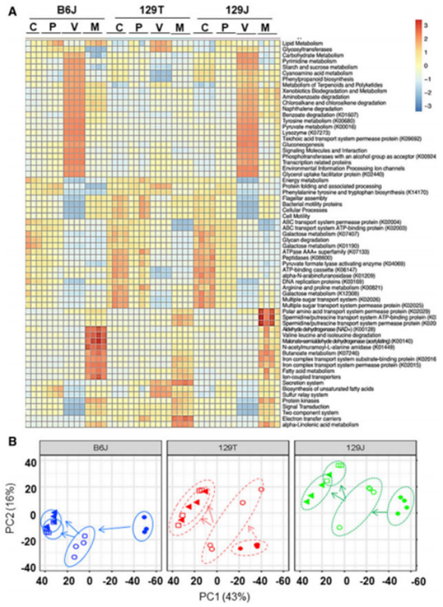 Figure 2 Gut microbiota alterations affect predicted functional metabolic pathways
Figure 2 Gut microbiota alterations affect predicted functional metabolic pathways
Diet, host genes, and gut microbiota interact to produce differential responses in plasma metabolites that help regulate metabolism and insulin resistance.
Shiho Fujisaka, et al. Diet, Genetics, and the Gut Microbiome Drive Dynamic Changes in Plasma Metabolites[J]. Cell Rep. 2018
 © Copyright 2015-2022 Suzhou PANOMIX Biomedical Tech Co.,Ltd
© Copyright 2015-2022 Suzhou PANOMIX Biomedical Tech Co.,Ltd